Posted on - February 15, 2012
By Venugopal Mallarapu, Director, Life Sciences Technology & Alliances at TAKE Solutions, Inc.
Signal Detection & Management
 In recent years, the life sciences industry has seen many product recalls and withdrawals from market due to safety concerns from patients and the resultant actions by regulators. Pharmacovigilance has become a more proactive process as opposed to a reactionary process in the past. So, what is a signal? CIOMS VIII (2010) [1] defines a signal as, “Information that arises from one or multiple sources which suggests a new, potentially causal association, or a new aspect of a known association between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action.” While the process of detecting and managing a signal could be complex, in its simplest form the following diagram depicts the process at a high level.
In recent years, the life sciences industry has seen many product recalls and withdrawals from market due to safety concerns from patients and the resultant actions by regulators. Pharmacovigilance has become a more proactive process as opposed to a reactionary process in the past. So, what is a signal? CIOMS VIII (2010) [1] defines a signal as, “Information that arises from one or multiple sources which suggests a new, potentially causal association, or a new aspect of a known association between an intervention and an event or set of related events, either adverse or beneficial, that is judged to be of sufficient likelihood to justify verificatory action.” While the process of detecting and managing a signal could be complex, in its simplest form the following diagram depicts the process at a high level.
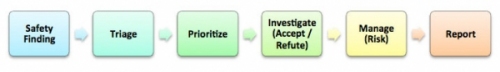
In the simplified process above, the methods employed to identify a safety finding (aka signal detection) are usually of two types:
- Qualitative: Manual review of all adverse events (pre-marketing, post-marketing, literature scanning, etc.)
- Quantitative: Applying scientific methods (statistical algorithms) and mine the safety databases (internal databases, the Adverse Event Reporting System (AERS), EudraVigilance, etc.)
The second part of the process is signal management, i.e., managing the identified safety finding until it is rejected or reported.
Signal Management Tools
The safety finding is usually captured in a document and managed through the process to ensure that the benefit-risk profile of the product is evaluated and appropriate impact is documented and reported. There aren’t many tools to manage the lifecycle of the safety observations available in the market. Large pharmaceutical organizations have invested heavily in building or procuring tools that can provide signal detection and management capability. However, most of the organizations are still using a manual process to capture and manage the lifecycle of the safety findings (signals).
Now, let us look at typical requirements for a tool that can be used for managing the signals:
- Forms: Ability to capture the information related to a signal in a form. Additionally, the tool should also provide ability to attach any documents and other artifacts required to the source of the signal so the information can be used in the triaging, prioritizing and investigation process.
- Workflow: As identified in the process above, the signal goes through various stages before it is either rejected or accepted. Once it is accepted it would be further investigated and any outcomes (risks) will be handled and closed. This entire process involves various stakeholders in the organization and will also entail specific Service Level Agreements (SLAs) for each step. So, any tool that manages the signals should be capable of defining a SLA-based workflow to handle the lifecycle.
- Alerts/Reminders: Since the process entails service levels, alerts and reminders during the process is a critical step to ensure that the signal is processed as per the agreed upon time limits.
- Validated: Since the system should adhere to GXP requirements, it has to be validated and compliant with regulations like Volume 9A and 21 CFR Part 11. This will ensure transparency and auditability of the system by regulators as well as other stakeholders.
- Integration: The information to be captured in the signal forms usually has attributes such as suspect product, individuals assigned to, etc. The tool should have the ability to integrate with other enterprise systems that usually manage such information. It should also be capable of integrating with downstream systems like a risk-management system that would be used to handle the risks identified as part of the signal evaluation and risk mitigation.
- Security: A system of such critical nature should be secure to prevent unauthorized access from internal or external stakeholders. It should also be capable of defining role-based security so not all users have access to all the steps of the lifecycle.
- Collaboration: The signal-evaluation process involves multiple stakeholders in the organization. The participants range from physicians to scientists to senior management. The tool should be capable of providing a collaborative environment where opinions and feedback could be expressed and communicated to a group of individuals. Also, the inferences and decision should be captured in the system.
- Dashboards and Reporting: This feature is required for senior management to be able to gauge the health of the signal-management process. The dashboards should be capable of providing a snapshot of aggregate data of all the signals and their status. Reports can also be used for review and audit purposes.
Microsoft SharePoint for Signal Management
According to a recent report titled “Using SharePoint for ECM – How well is it meeting expectations” [2] published by the consulting firm AIIM Inc., Microsoft SharePoint is a very popular collaboration and content-management platform adopted by many organizations across the globe. Life sciences organizations are no exception to this trend. In recent years, SharePoint has seen rapid growth in even areas that require more cautious adoption of new technology due to the impending regulatory requirements (e.g., Volume 9A or 21 CFR Part 11). However, due to the efforts from Microsoft as well as other IT and life sciences organizations to help customers adopt SharePoint in a regulated environment [3], it is being adopted in areas where it requires validation.
Since many life sciences organizations have already invested into SharePoint, in the context of the requirements for a signal management tool, it can also be leveraged for the purpose of managing signals. The most recent release of SharePoint, i.e. 2010, provides the following major features that are relevant to the requirements identified above:
- Forms [4]: SharePoint provides multiple options to create and use forms. The options available include Microsoft Word-based forms to InfoPath to Microsoft Access to ASP .NET Web Forms. Depending on the complexity of the forms, i.e. the nature of controls required, data source integration, etc., the technology can be selected.
- Workflow [5]: SharePoint provides flexible workflows that can be easily deigned and managed. Teams can use SharePoint Designer to develop and maintain simple workflows or can also use Microsoft Visual Studio to develop more complex workflows. Given the nature of the process defined above, in most cases a simple SharePoint designer-based workflow should be capable of handling the signal management process.
- Workflow Actions [6]: For the requirements to send out alerts and notifications as well as to setup service levels associated with each step, workflow actions can be leveraged. The extensive list of actions available will be capable of providing all the flexibility required to define and manage the service levels associated with each step, as well as provide approval and feedback mechanisms required at each step of the way.
- Security [7]: SharePoint provides multiple authentication and authorization mechanisms. Depending on the type of access required for the signal management, the corresponding authentication and authorization method can be selected.
- Business Connectivity Services [8]: Microsoft provides Business Connectivity Services (BCS) as part of the SharePoint Server infrastructure that can be leveraged to connect to external data sources through web services, database and .NET assemblies from within SharePoint 2010 application. This component will fulfill the need for integration with other enterprise systems to source data.
- Business Intelligence [9]: SharePoint provides multiple tools like PerformancePoint Designer, Visio and Excel to design and develop reporting and dash boarding. Depending on the complexity of the dashboards and/or reports, appropriate technology can be used.
- Audit Logs [10]: SharePoint provides audit log capability to track the activity of all the users using the system. The granularity of the tracking can be set up depending on the level of tracking required from a compliance perspective.
- Electronic Signatures: Electronic signatures can be captured as part of the workflow whenever a specific action is performed by the stakeholder. Also, third-party tools are available if one wants to implement sophisticated digital signature mechanism. The 21 CFR Part 11 guidance from Microsoft also provides additional details on various aspects of fulfilling compliance requirements.
Conclusion
In essence, a life sciences organization can maximize the investments made in Microsoft SharePoint by adopting it to automate the safety signal management workflow. As illustrated, SharePoint has all the necessary components required to support such an effort. Also, depending on the complexity of the workflow, the solution can be simple to complex and can be accomplished through configuration and customization of SharePoint, respectively.



